An atom loses an electron when it gains or shares one with another atom. This process is called an electron transfer and it happens in many different ways. Some of the ways atoms lose electrons include radioactive decay, photosynthesis, and chemical reactions.
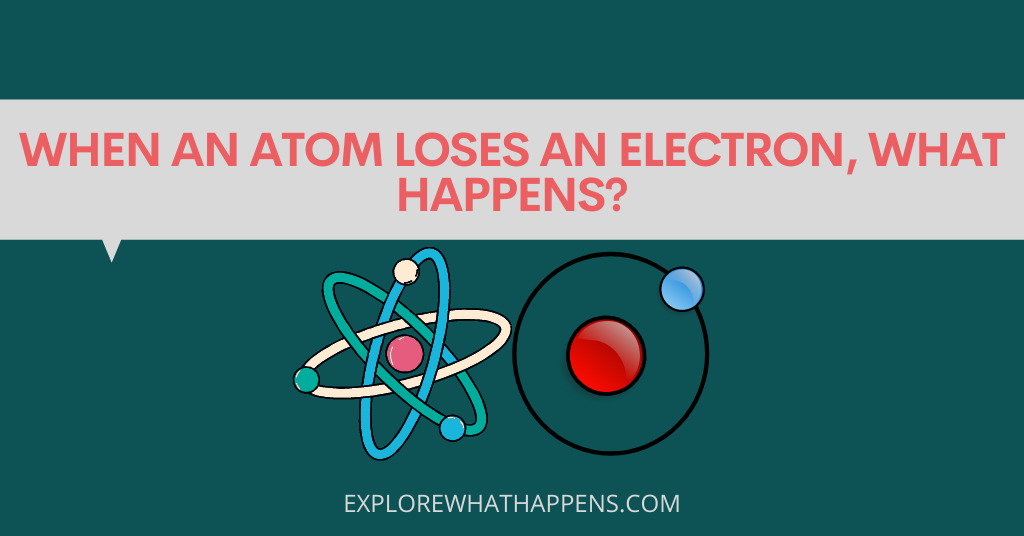
When an atom loses an electron, it becomes a different element. This process is called oxidation and it happens in two ways: spontaneously and through a reaction. Oxidation occurs when the free electron jumps from the atom to another molecule, ion, or nucleus. Spontaneous oxidation is when the electron jumps away on its own accord. Reactions occur when two atoms combine to form a new element.
An electron is a subatomic particle that is associated with an atom. It is the basic unit of charge in an atom, and is attracted to the nucleus of the atom. A proton is a positive particle of charge that is associated with a neutron. Neutrons are electrically neutral particles. The protons in the nuclei of most atoms are positively charged and the electrons are negatively charged. The protons are held tightly together by the strong nuclear force, while the electrons orbit the nucleus.
What science tells us about the losing and gaining of electrons:
When an atom loses an electron, it becomes positively charged. A positive ion is created, and the nucleus attracts the positively charged particles. An electron is not lost when an atom gains an electron; the atom simply has a different number of electrons.
Ions can lose or gain electrons. This can occur through chemical reactions. Electrons can also move from one atom to another during radioactive decay.
When an atom loses an electron, the atom gains a new electron. When an atom gains an electron, the atom loses an electron. An atom can also become a molecule by losing or gaining electrons.
There are many ways of classifying an atom. There are three main ways to classify an atom:
Isotopes:
An isotope is an atom of the same element with a different number of neutrons. For example, the isotopes of oxygen have different numbers of neutrons. Oxygen-16 has 8 neutrons, oxygen-18 has 10, oxygen-17 has 11, and oxygen-16 has 12. An isotope can have the same number of protons and neutrons. For example, oxygen-16 and oxygen-18 are both composed of two protons and eight neutrons. Isotopes are a type of homogeneous chemical element.
Isotopes are classified according to the number of neutrons in the atom. Isotopes of an element can be distinguished by the mass of the atom.
Isotopes with the same atomic number (number of protons) and atomic mass are considered to be the same element.
A nucleus of a nonradioactive isotope is stable. Radioactivity is the process of an unstable atom changing over time.
Nuclear fission:
When a heavy atom like uranium-235 decays, it splits into smaller, lighter atoms. These atoms are called isotopes. The isotope with the lowest number of neutrons is called the fission product. The fission product is unstable and radioactive.
When a uranium-235 atom decays, it emits an alpha particle (helium) and a proton. The uranium atom loses an electron to become a positively charged ion. The alpha particle has a mass of 4 MeV. The proton has a mass of 1.8 MeV.
Fission products are produced by a nuclear reaction.
When the nucleus of a uranium-235 atom is split, it is transformed into several fragments (nuclei). When the nucleus of a uranium-238 atom is split, it is transformed into two fragments (nuclei).
The nuclear fission process produces more energy than the decay of a radioactive element.
Nuclear fission is a process that occurs in the nucleus of the uranium-235 atom. It is the process that releases energy and causes radioactivity.
The fission process can occur naturally, and the process can be triggered.
When a nuclear fission process occurs, the mass of the nucleus increases. When a uranium-235 atom decays, the mass of the resulting nucleus decreases.
Nuclear fission of an atom produces more energy than the decay of a radioactive element.
When a uranium-235 atom decays, the nucleus becomes less massive. When a uranium-235 atom decays, the resulting nucleus becomes less massive.
Nuclear fission is the process of splitting the nucleus of a heavy atom such as uranium-235, uranium-238, or plutonium.
Nuclear fission of an atom produces more energy than the decay of a radioactive element.
In a nutshell, when an atom loses an electron, it becomes a positively charged ion. This can have serious consequences for the atom, as it can no longer hold onto its electrons and atoms around it can start to pull them away. This can cause the atom to become unstable and potentially release energy in the form of radiation. For this reason, it is important to understand how atoms lose electrons and what happens when they do.